Pilot studies are essential for modern research. They help scholars test feasibility, refine tools, and identify potential challenges. However, pilot studies are not final research. Treating them as conclusive undermines credibility, misguides policy, and damages trust in science.
This article explains the role of pilot studies, highlights common pitfalls, and offers practical solutions for using them correctly.
What Is a Pilot Study?
A pilot study is a small-scale preliminary investigation conducted before a full research project. Its main goals are to:
- Test feasibility of methods
- Refine research instruments
- Identify logistical or methodological challenges
- Explore data patterns without drawing final conclusions
According to Thabane et al. (2010, BMC Medical Research Methodology), pilot studies should be designed with clear objectives: refining methods, not proving hypotheses.
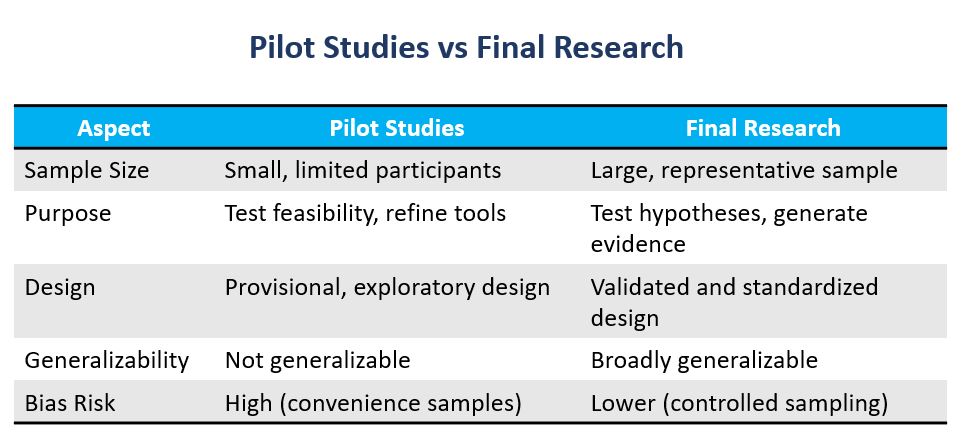
Why Pilot Studies Are Not Final Research
1. Limited Sample Size
Pilot studies usually involve small groups. Small samples lack statistical power. They cannot support generalizable claims (Leon et al., 2011).
2. Exploratory Purpose
The goal of a pilot is refinement, not proof. Treating exploratory results as final evidence leads to premature claims and weak science.
3. Design Limitations
Pilot studies often test instruments, sampling, and protocols in early stages. These provisional designs are prone to bias, measurement error, and inconsistency.
4. Bias and Convenience Sampling
Due to cost and time constraints, pilots often use convenient samples. These groups rarely represent the wider population, which weakens generalizability.
Why Misusing Pilot Studies Matters
When scholars or policymakers misuse pilot studies, serious issues arise:
- Overstated Claims: Credibility of research is reduced.
- Misguided Policy Recommendations: Policymakers may act on weak evidence.
- Lost Trust: Reviewers and funders may question integrity.
- Stalled Scientific Progress: Without follow-up studies, evidence remains shallow.
Best Practices for Using Pilot Studies
At Research & Report Consulting, we recommend:
1. Refinement Over Results
Focus on improving tools, protocols, and logistics rather than producing results.
2. Transparent Reporting
Clearly state that pilot findings are exploratory and non-generalizable.
3. Bridge to Full Research
Use pilot data to design robust, large-scale studies with strong validity.
4. Avoid Over-Claiming
Report limitations upfront. Protect your credibility and ensure reviewers understand the scope.
Final Thought
Pilot studies are invaluable—but they are practice runs, not the finish line. Their true value lies in preparing the ground for credible, large-scale research. When pilot studies are misused as conclusive evidence, both science and policy suffer.
Question for Readers:
Have you ever seen pilot studies misrepresented as final research? What impact did it have? Share your thoughts below!
References
- Thabane, L., et al. (2010). A tutorial on pilot studies: the what, why and how. BMC Medical Research Methodology, 10:1.
- Leon, A. C., et al. (2011). The role and interpretation of pilot studies in clinical research. Journal of Psychiatric Research, 45(5), 626–629.
- E van Teijlingen, E., & Hundley, V. (2001). The importance of pilot studies. Social Research Update.

